New Study in The FASEB Journal: Proper Folding is Important for a Protein Associated with Seizures
Monday, June 9, 2025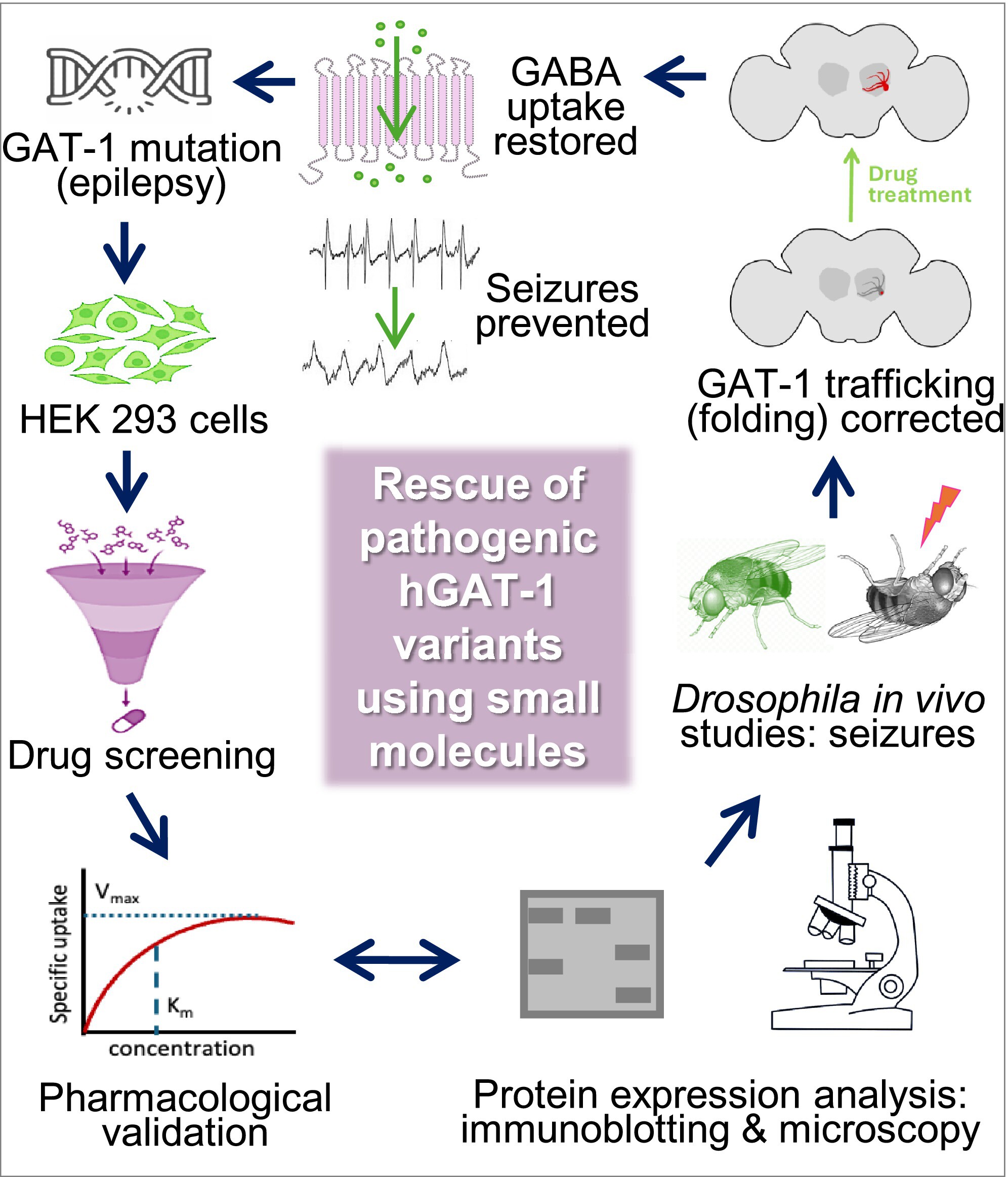 Just as folds are important in the ancient art of origami, they are also vital for the function of many proteins. Mutations in a protein associated with conditions such as seizures and autism spectrum disorder prevent it from folding correctly and hinder its movement to the cell surface, where it would normally do its job. Certain small molecules can reverse this effect, opening the door to the development of new treatments, according to a recent study published in The FASEB Journal.
Just as folds are important in the ancient art of origami, they are also vital for the function of many proteins. Mutations in a protein associated with conditions such as seizures and autism spectrum disorder prevent it from folding correctly and hinder its movement to the cell surface, where it would normally do its job. Certain small molecules can reverse this effect, opening the door to the development of new treatments, according to a recent study published in The FASEB Journal.
Human GABA transporter 1 (hGAT1), encoded by the SLC6A1 gene, helps brain cells take up the inhibitory neurotransmitter GABA from synapses. However, when hGAT1 doesn’t work properly or doesn’t get to the cell’s plasma membrane, neurodevelopmental conditions can occur. Many SLC6A1 mutations are associated with these types of conditions in humans.
Recently, Ameya Kasture, Nikita Shah, Sonja Sucic, and colleagues based at the Medical University of Vienna in Austria showed that 15 of the disorder-causing hGAT1 mutations have negative effects on the protein’s function. After the mutant proteins are made, they do not fold properly and tend to accumulate within the cell in the endoplasmic reticulum instead of moving on to the plasma membrane. In the current study, the team focused on two mutations in a highly conserved glycine at amino acid position 443 that are associated with childhood epilepsy. The mutation G443D has aspartic acid instead of glycine, whereas G443V has a valine at that position.
In vitro tests showed that some of the G443D mutant protein makes it to the plasma membrane, though some of it also becomes trapped within cells. In contrast, G433V mutant protein is stuck in the endoplasmic reticulum and does not reach the plasma membrane. Neither mutant can take up GABA. Small molecules that help proteins fold into the correct shape reverse these effects, however. Glycerol increased GABA uptake in cells with the G443D mutation, and 4-phenylbutyrate increased uptake in cells with the G443V mutation. Treatment with these small molecules also boosted the ability of mutant—and wild type—proteins to reach the plasma membrane.
The researchers then studied the mutations in vivo in fruit flies and found similar localization defects. When exposed to heat stress, both types of mutant flies had seizure-like movements. However, eating food supplemented with 4-phenylbutyrate reduced the number of seizure episodes and corrected the localization of G443V mutant proteins in flies. Glycerol treatment slightly reduced the seizure activity in both mutants.
“Our current study provides new evidence that GAT-1 mutations can impair protein trafficking, function, or both, underscoring the need for a systematic mutation-specific evaluation to understand individual SLC6A1-related symptoms,” say the authors. “The results offer valuable insights into the pathophysiology of GAT-1-related disorders and suggest new avenues for potential therapeutic intervention.”
Funding: Austrian Science Fund
Read the full article, “Rescue of epilepsy-associated mutations of the highly conserved glycine residue 443 in the human GABA transporter 1,” published in The FASEB Journal.